Catalog of Regulatory Science Tools to Help Assess New Medical
€ 32.50 · 4.9 (118) · Auf Lager


Medical Device Trials at SCOPE Summit

Articles Precision Nanomedicine

Interdisciplinary Academic Framework for Medical Regulatory Science|What is RS?|Institute for Medical Regulatory Science
Considerations for diagnostic COVID-19 tests
How FDA Regulates Artificial Intelligence in Medical Products

Medical Device Innovation Consortium
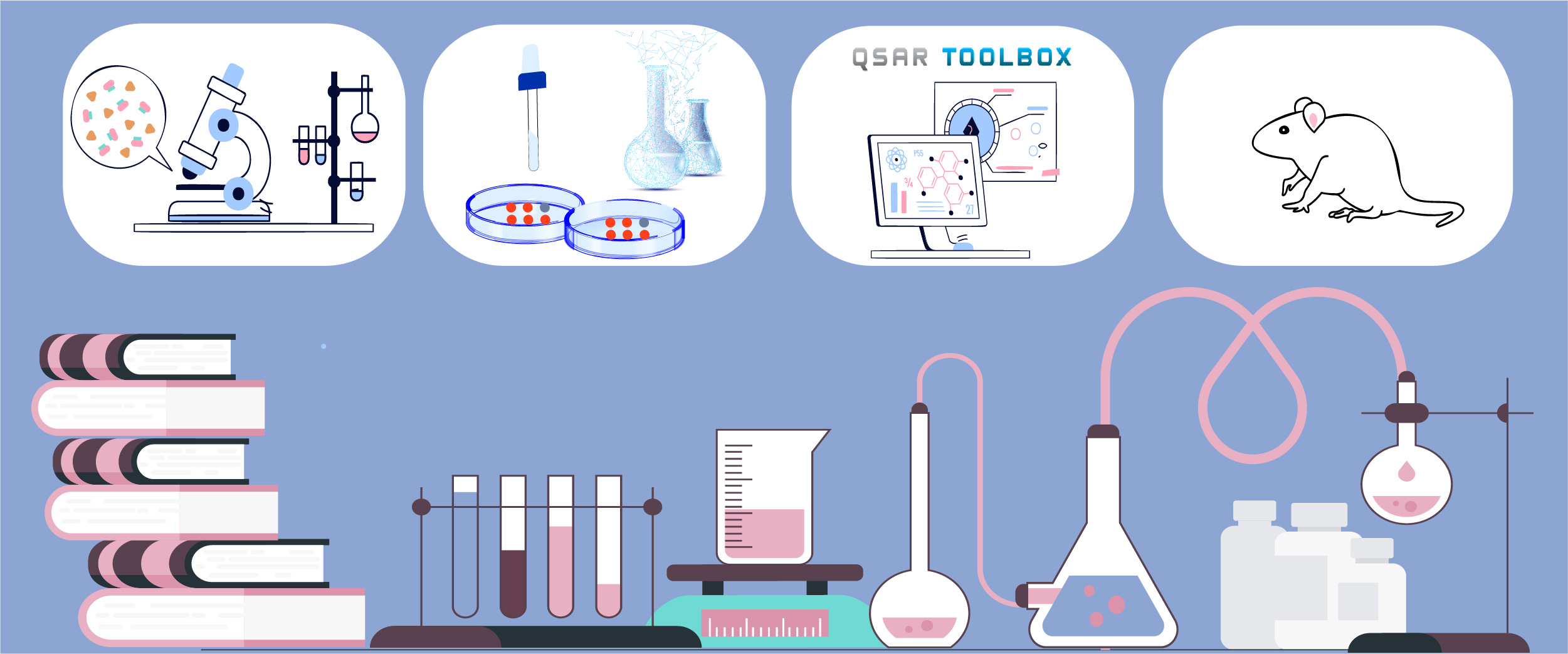
Integrated Approaches to Testing and Assessment (IATA) - OECD

FDA Adds New Tools for Evaluating Medical Devices
Overview of the Regulatory Pathway for In Silico Testing of Medical Devices

Want to See the Future of Digital Health Tools? Look to Germany.
Pharmaceutical Quality Management System (QMS) - SimplerQMS

Regulatory Considerations on the use of Machine Learning based tools in Clinical Trials

Regulatory Review of Novel Therapeutics — Comparison of Three Regulatory Agencies
6 Regulatory Pathways to Bring Your Medical Device to Market
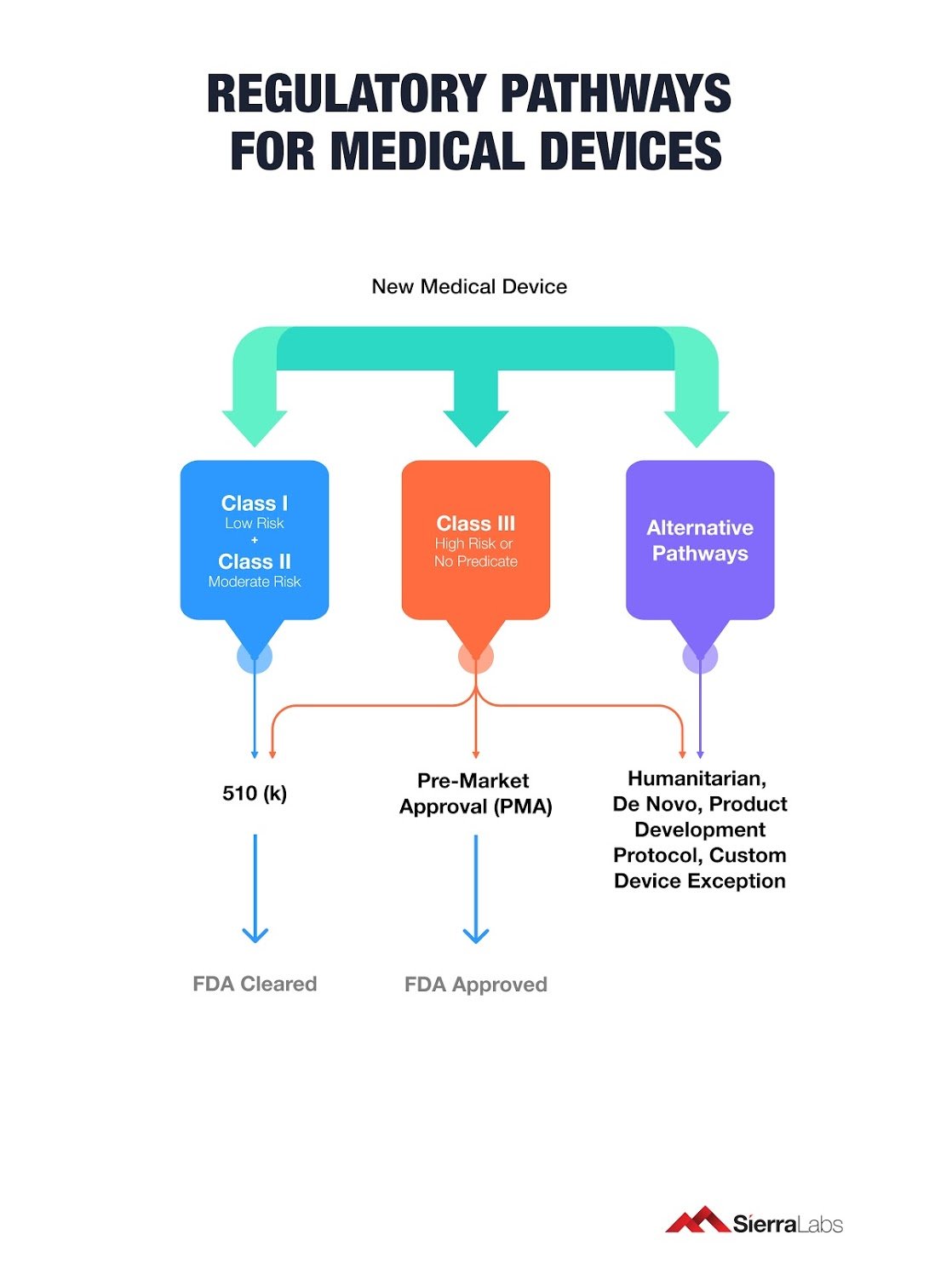
6 Regulatory Pathways to Bring Your Medical Device to Market






.jpg)