cobas® SARS-CoV-2 & Influenza A/B Test
€ 18.99 · 4.8 (385) · Auf Lager

Multi-center nationwide comparison of seven serology assays reveals a SARS- CoV-2 non-responding seronegative subpopulation - eClinicalMedicine

Roche Covid-19 and Influenza A/B Test Receives EUA

Roche 09211101190 - McKesson Medical-Surgical

Evaluating the Ability to ID (COVID-19) NOW: a Large Real-World Prospective Evaluation of the Abbott ID NOW COVID-19 Assay

Roche 09211101190 - McKesson Medical-Surgical

FDA authorized molecular point-of-care SARS-CoV-2 tests: A critical review on principles, systems and clinical performances - ScienceDirect

Roche gets emergency approval for combination COVID-19, flu A/B test
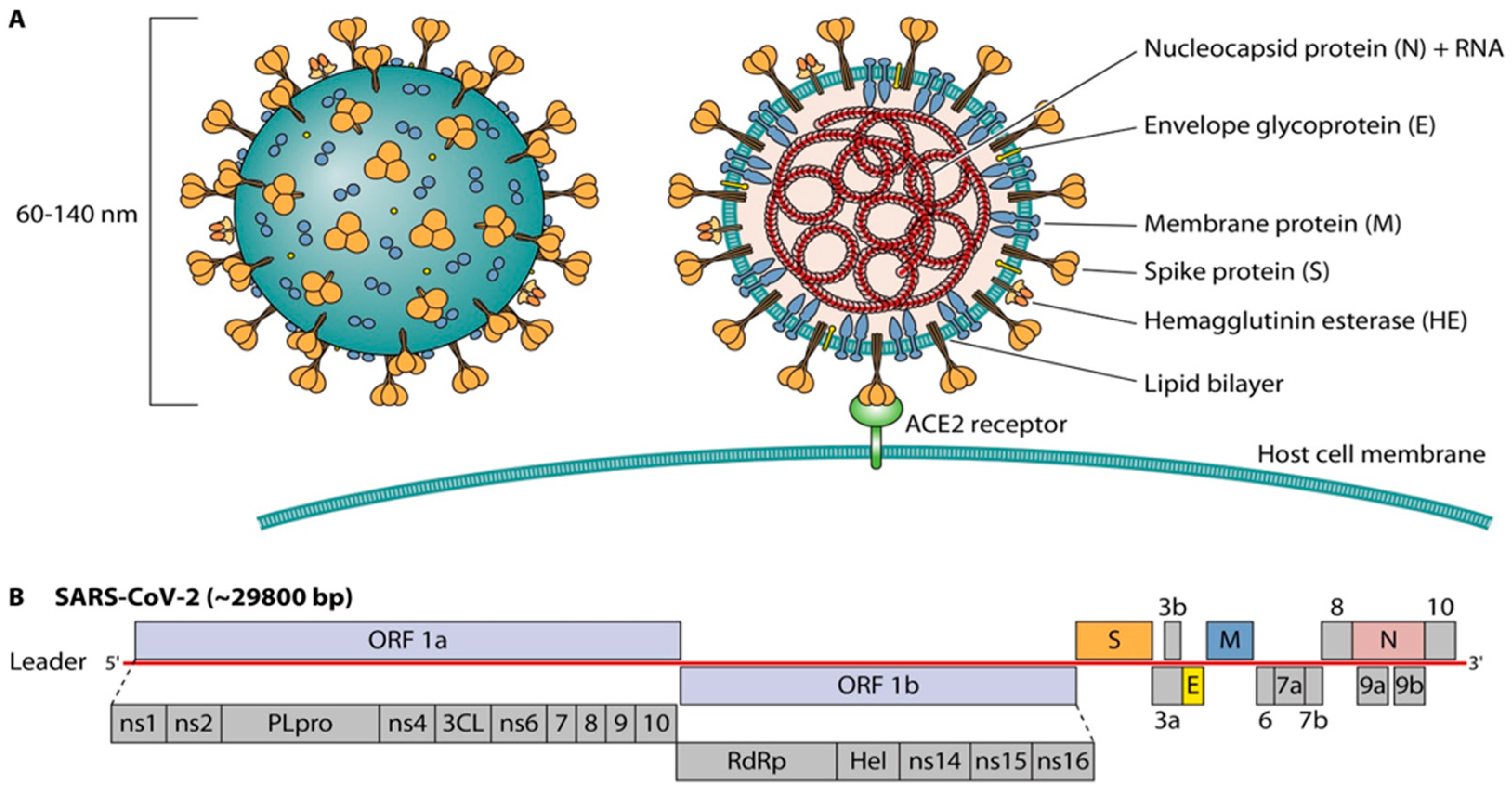
Diagnostics, Free Full-Text
East Nashville Family Medicine - RAPID COVID-19 PCR TESTING AVAILABLE!!! We were temporarily out of stock last week but have received a new shipment of the Cobas SARS-CoV-2 & Influenza A/B Nucleic
Roche su LinkedIn: SARS-CoV-2 & Flu A/B Rapid Antigen Test
The cobas SARS-CoV-2 & Influenza A/B nucleic acid test is a multiplex real-time polymerase chain reaction (PCR) test that detects and differentiates

Roche Diagnostics cobas Liat SARS-CoV-2 + Influenza A/B Control Kit

Performance Evaluation of the Microfluidic Antigen LumiraDx SARS-CoV-2 and Flu A/B Test in Diagnosing COVID-19 and Influenza in Patients with Respiratory Symptoms
![]()
FDA greenlights combination at-home test for COVID and influenza in children as flu season approaches
Two Years into the COVID-19 Pandemic: Lessons Learned